
Dr. Maho Hamasaki, Associate Professor, Graduate School of Frontier Biosciences
"An inclusive research environment fuels passion and drives excellence in science"
Dr. Hamasaki’s expertise is cellular biology, dedicating herself to unraveling the mechanisms that initiate autophagy—a process where cells degrade components within themselves.
She completed her secondary and higher education in Canada and joined Professor Yoshinori Ohsumi’s laboratory during her doctoral studies. Her decision was driven by a pivotal moment at a scientific conference, where she encountered a poster presentation from the lab. At the time, she did not know about autophagy nor was she familiar with Professor Yoshinori Ohsumi, who later succeeded in elucidating the molecular mechanisms of autophagy, earning him the Nobel Prize in Physiology or Medicine in 2016. The people and atmosphere in the lab were encouraging and built her foundation as an established researcher. Dr. Hamasaki was selected among thirteen female researchers around the world for “Women in Science” by Nature Cell Biology.
Pursuing the mechanisms of autophagosomes
Autophagosome membrane, which sequesters intracellular molecules and structures within its membrane-bound structure during autophagy, is the focal point of Dr. Hamasaki's research.
The primary function of autophagy is to maintain cellular metabolism and provide nutrients for survival during starvation by breaking down some of its own components. This process begins with the formation of an "autophagosome," which encapsulates targeted cellular materials.
"First, a flat membrane called the isolation membrane appears in the cytoplasm. As it elongates, it engulfs the target. Eventually, the ends of the isolation membrane fuse, completing the double-membrane structure of the autophagosome, which is composed of lipid bilayers. The autophagosome then fuses with lysosomes—organelles responsible for degradation—to digest its contents. This process is highly unique, as autophagosomes appear only when needed, perform their function, and disappear afterward," explains Dr. Hamasaki.
Since her doctoral studies, Dr. Hamasaki has focused on the membrane dynamics of autophagosomes. "I have been working to elucidate the mechanisms, including the origin, of how, when, and where the double membrane that encapsulates the targets is formed," she says.
A groundbreaking discovery made possible through live imaging
Dr. Hamasaki was fascinated by imaging during her PhD study and joined the European Molecular Biology Laboratory to study the technique as a postdoc. This passion led to her success in capturing the dynamic positional relationships between the endoplasmic reticulum, mitochondria, and autophagosomes with remarkable accuracy by using three-color simultaneous live fluorescence imaging.
In 2013, her groundbreaking study published in Nature demonstrated that autophagosomes are formed at the contact sites between the endoplasmic reticulum (a membranous organelle within the cytoplasm) and mitochondria. This discovery marked a significant contribution to the field and garnered widespread recognition.
She has continued to delve into the mechanisms involved in autophagosome formation through her research, specifically regarding what factors are involved in the formation of autophagosomes at the contact sites between the endoplasmic reticulum and mitochondria. She believes that uncovering the molecular mechanisms behind the initiation of autophagy can advance our understanding of autophagy while also providing insights into the onset and progression of conditions such as aging-related disorders and neurodegenerative diseases.
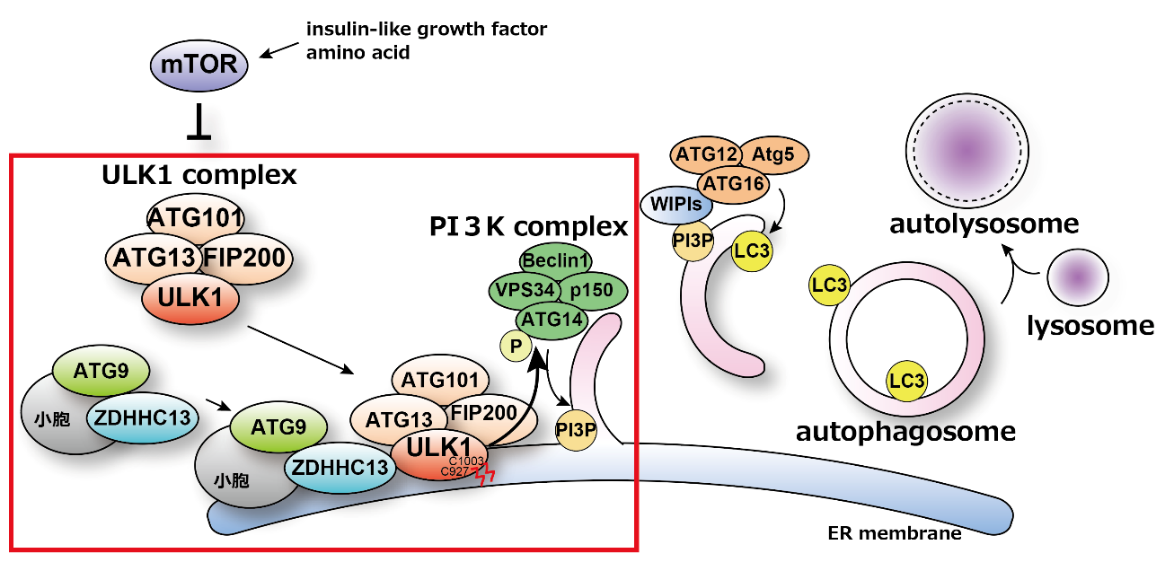
In 2024, Dr. Hamasaki’s lab unveiled a new mechanism crucial for the initiation of autophagy. The research group previously demonstrated that autophagosome formation occurs on the endoplasmic reticulum membrane near mitochondria and identified the PI3K complex, an autophagy-related protein group, as essential for this process. The activity of the PI3K complex is regulated by the ULK1 complex, which is known to translocate from the cytoplasm to the endoplasmic reticulum membrane—the site of autophagosome formation—at the onset of autophagy. However, the mechanisms and significance of this translocation remained unclear.
In their recent findings, the team discovered that the enzyme ZDHHC13 palmitoylates ULK1, enabling its localization to autophagosome formation sites. This palmitoylation of ULK1 also promotes the phosphorylation of ATG14L, a component of the PI3K complex, leading to PI3K activation and the initiation of autophagy.
An ardent passion for research and fostering the next generation
“What you do has to be enjoyable and something you’re truly passionate about; otherwise, you can’t keep doing it. I’m the type who likes to observe and think for myself, so I just love experimenting. I’m also involved in nurturing the next generation of researchers, and seeing graduate students working hard makes me incredibly happy.”
Text: Saori Obayashi/Edit: Christopher Bubb