
Professor Koji Nishida, Department of Ophthalmology, Graduate School of Medicine
"Human metaverse medicine: The birth of a new science"
Professor Kohji Nishida was the first in the world to prepare corneal epithelial cell sheets using iPS cells and graft them into patients with corneal diseases. He has since gone on to make many globally acknowledged achievements, including two-dimensionally reproducing the development of cells that constitute the eye using iPS cells and the discovery of vascular endothelial stem cells. Now, Professor Nishida has embarked on the grand new challenge to create a new scientific field: Human Metaverse Medicine.
Leading a world-class research center in Japan
Professor Nishida is the leader of Osaka University’s Premium Research Institute for Human Metaverse Medicine (PRIMe), which has been selected as one of Japan’s most prestigious research centers in the 2022 World Premier International Research Center Initiative (WPI) sponsored by the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Human Metaverse Medicine is a new science that integrates two academic fields: human organoid-based biomedical science and information and mathematical sciences. Through this endeavor, PRIMe aims to elucidate disease mechanisms, predict disease onset and progression and individual drug medicine, and develop individualized prevention methods and curative treatments by reproducing life phenomena and disease processes in a virtual space known as the human metaverse, creating what are known as “biodigital twins.” Human organoids (miniature organs made from iPS cells and other sources) constructed from healthy, pre-symptomatic, or diseased individuals will play a critical role in these biodigital twins.
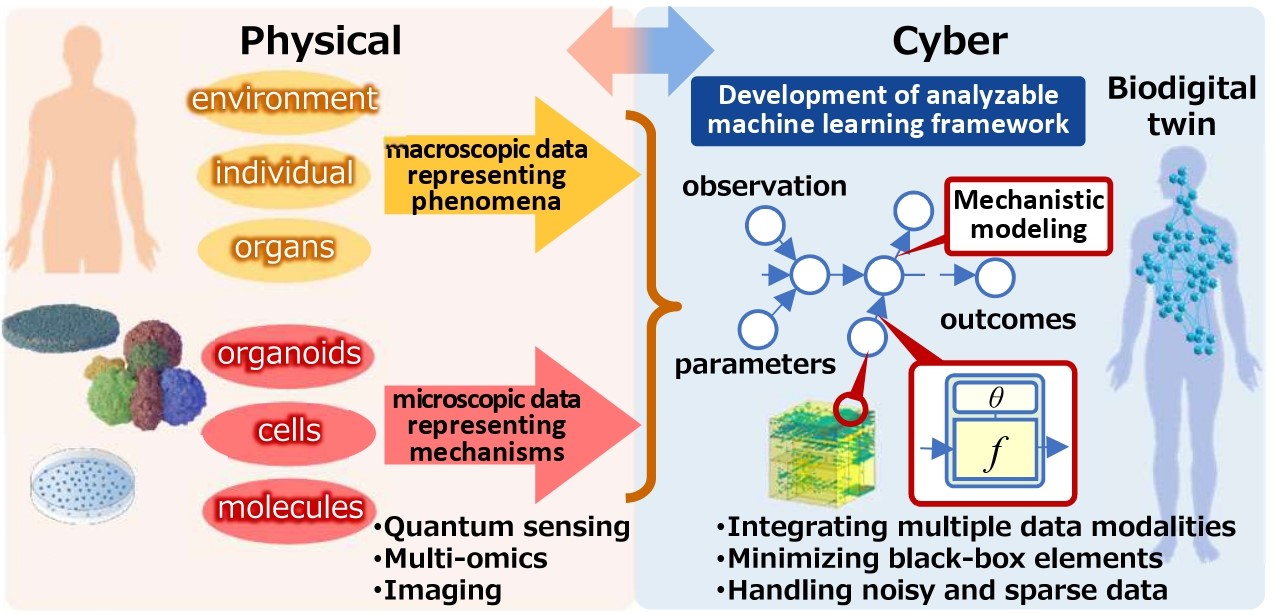
Fig. Creation of platform to develop biodigital twin
A pioneering powerhouse in ophthalmology
Professor Nishida has already been developing organoid research since the late 2010s based on accumulated findings and technologies through studies using iPS cells. The pioneering research of his team was published in Nature in 2016, research results which stunned the world. They succeeded in developing cells in a regular order from the posterior to the anterior parts of the eye using iPS cells and arranging them into a laminar structure. They were cells arranged concentrically in 4 layers and were designated as the self-formed ectodermal autonomous multi-zone (SEAM). SEAM, which could really be called a two-dimensional “eye,” is an achievement with a concept close to that of the organoid. In their most recent research, Professor Nishida’s group has succeeded in the development of a method for the generation of three-dimensional (3D) iPS-derived organoids that model the tear duct (also known as the lacrimal gland), leveraging their SEAM.

The next stage of a newly discovered field
With those outstanding technologies and accumulated knowledge, PRIMe will promote the projects in a global alliance with advanced research institutes, including NTT Basic Research Laboratories, RIKEN Center for Advanced Photonics, Cincinnati Children’s Hospital Medical Center, Curie Institute, and Stanford University and University College Dublin.
At PRIMe, operations such as genome editing are performed on organoids to make it possible to reproduce the onset of disease much faster than we can achieve in reality.
The changes that occur in the organoid are then recorded on a variety of scales on a broad temporal axis, stretching from milliseconds to several months. This information, together with conventional human body data, is then sent to cyberspace.
Relationships among the data obtained are structured through newly developed relational data analysis and modelled using uniquely developed mathematical modelling modules, making it possible to convert and complement information and draw inferences.
PRIMe constructs a biodigital twin for each organ in which relationships between data are expressed in this way and then increases the amount of information to improve accuracy, while at the same time evolving the biodigital twin from the organ level to the organ network level and the human body level.
Because the biodigital twin comprehensively models information at various levels ranging from the individual to organ, cell, and molecule, it can determine at the cellular or molecular level the cause of abnormality in the living body by tracing the data network. It can also predict organ dysfunction and individual drug response, which can then be applied to the development of new drugs as well.
(Source: https://youtu.be/ZjNbnUMEQ7A)
With his ceaseless passion for discovering the unknown, Professor Nishida continues his trailblazing journey until the baton is ready to be handed the next generation.
Text: Saori Obayashi/Edit: Christopher Bubb