
Creating Revolutionary Metal Complexes that Defy Common Wisdom in Materials Science

Creating new types of complexes to lead to innovation
Complexes are compounds that contain metals. According to Professor Konno, “Metallic ions don’t exist by themselves in water, mineral or living things, but rather, they combine with organic or inorganic matter to form complexes.”
He added, “For example, the hemoglobin protein is a protein that forms a complex with iron molecules. Cisplatin, which is effective at treating cancer, is also a platinum complex.”
A coordination complex consists of an ion, which is usually metallic, and a surrounding array of bound molecules or ions. Metal complexes find wide application in various fields from medicine to chemical engineering as functional materials, such as organic EL.
As the functions of complexes change depending on which materials are bonded to metallic ions, fundamental research on complex chemistry is essential for creating innovation.
Professor Konno tries to "defy common wisdom in materials science through coordination molecular technology.” Based on the understanding of properties of metal complexes by examining the state of bonds, he tries to create complexes with new functions.
All of the elements on the periodic table are within the scope of his research. “Complex chemistry is chemistry of all elements. I always say that I’m ‘traveling the periodic table.’”
His goal is to “create a totally new concept of matter”
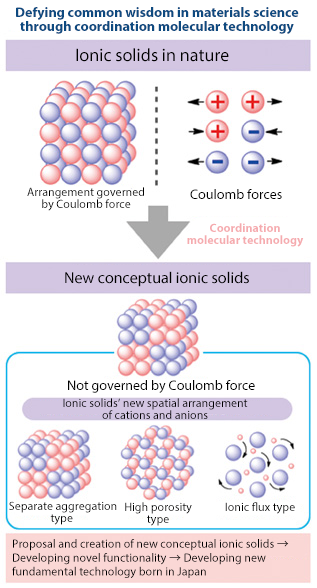
Complex chemistry is closely connected to a variety of fields. Professor Konno commented on his research goals, saying, “I want to create a totally new concept of matter."
He continued, "I want to be the first in the world to create new conceptual metal complexes-based ionic solids, in which the spatial arrangement of cations and anions are not governed by Coulombic force (electrostatic force).”
He is proposing ionic solids with three types of arrangement of ionic species: (1) ionic solids with separate aggregation of cations and anions, (2) ionic solids with a high porosity, and (3) ionic solids with AC conductivity due to an ionic flux.
An accident in 2010 drove him to study the complex structures that might lead to innovation.
“When I had performed x-ray analysis on a complex that a student had synthesized for some other purpose, I just happened to look at the overall structure of the complex, and a crystal structure that I had never seen before appeared right before my eyes. At that time, I was convinced that it was possible to create ionic solids based on a concept that is completely opposite from conventional ones.”
Unique research will be recognized when the time comes

The challenge of this research is figuring out what combination of metal ions is best to design a complex.
I increased the number of coordinate bonds in a step-by-step manner from a coordination compound with just one metal to a coordination compound with 116 coordinate bonds. It took some 30 years since I started this research as a student.
Professor Konno also mentioned an important aspect of research in general, saying, “You must carefully and steadily continue your research without being affected by trends. While you are pursuing your own field, your research may occasionally come to correspond with today’s trends,” he said.
"At the Konno Lab, information sharing and communication between members, including students, is highly valued. The lab holds a small meeting twice a day. We also have a biannual trip and bowling competitions. These are great chances for members to grow closer.”
Coordination chemistry was started by the late Professor TSUCHIDA Ryutaro at Osaka University. Professor Konno, who studied under a pupil of Professor Tsuchida himself, said with a smile, “Even research that seems far off from practical application at first could eventually come to fruition one day. That’s the beauty of research.”

• Takumi KONNO
A 1980 graduate of the Department
of Chemistry, University of Tsukuba, Professor Konno received his
doctorate in 1985 in the Graduate School of Chemistry, University of Tsukuba. He
became a research fellow in the Department of Chemistry at the
University of Tsukuba in 1985, a postdoctoral research fellow at the Department of
Chemistry, University of Cincinnati in 1986, an assistant professor in the Department of Chemistry, University of Tsukuba in 1987, and a lecturer at the same
department in 1994. After serving as an Assistant Professor in 1997 and a Professor
in 1998 in the Department of Chemistry at Gunma University, he began at his
current position as a Professor at the Graduate School of Science, Osaka
University in 2000.