
Professor Kei Ohkubo, Institute for Advanced Co-Creation Studies
"Groundbreaking chemical reactions lead to new eco-friendly methods for liquid fuel production and plastic surface oxidation"
Methane, the main constituent of natural gas, is a substance familiar to us. Although it is seen as a promising energy source, the storage and transportation are costly. Converting methane into methanol, a type of liquid fuel, greatly enhances its usefulness. Dr. Ohkubo succeeded in the world’s first methanol synthesis under ambient conditions with the use of oxygen in the air to react with methane in a solvent under photoirradiation [1].
A series of coincidences leads to an epoch-making reaction
It is difficult to oxidize methane, which is chemically inert, to methanol. Conventionally, industrial methanol synthesis has been carried out under high-pressure and high-temperature conditions, emitting lots of CO2 with a limited yield of methanol. Innovative methods that overcome these shortcomings remained a challenge for chemists around the world.
Dr. Ohkubo’s discovery of a breakthrough molecule came by chance when he was asked to investigate the mechanisms of a new type of deodorant. His intuition told him that the product contains a strong oxidizing agent that decomposes organic matter, the source of the offensive odor. The main component of the product? Chlorine dioxide (ClO2).
“A colored substance tempts me to throw light on it. Photoirradiation of a solvent in which yellow ClO2 and methane are dissolved produced methanol. I was astonished.” Luckily, Dr. Ohkubo had perused a leaflet on a fluorous solvent. The fluorous solvent dissolve gaseous substances well and do not mix with water. “That was the agent I was looking for!”
ClO2 activated under photoirradiation causes methane gas dissolved in the solvent to react with oxygen, which forms methanol and formic acid. The reaction occurs at ambient temperature and pressure. The yields of methanol and formic acid are 14% and 85%, respectively, with a methane conversion of c.a. 100% without the concomitant formation of CO2. Considering the poor yield for conventional, less eco-friendly methods, he finally achieved a goal that eluded him for decades.
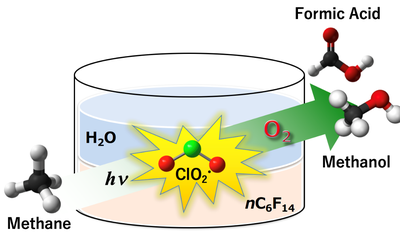
Figure: Chlorine dioxide (ClO2) was found to act as an efficient oxidizing agent in the aerobic oxygenation of methane to methanol and formic acid under photoirradiation.
Now LED-ing the way – a clean and convenient method to oxidize plastic surfaces for industry
Earlier this year, Dr. Ohkubo also successfully oxidized the surface of polypropylene (PP), one of the most widely used plastics, by using an LED-light-driven reaction, garnering attention for its prospective uses in the polymer industry [2].
The process uses radicals to make it possible to oxidize the plastic surface. The surface of PP bristles with methyl groups (–CH3 ), which constitute the side chains of the polymer. The strong C–H bonds in methyl groups make PP an chemically unreactive and stable material, which is actually necessary for many purposes. However, these bonds can be cleaved by the highly reactive chlorine dioxide, ClO2.
Previous methods for oxidizing olefinic polymers such as PP and polyethylene were either poorly controlled or highly polluting. The new process is thus the first clean and convenient solution to this problem, and may prove to be a valuable industrial tool in the customization of synthetic plastics.
You can read more about this study at: https://resou.osaka-u.ac.jp/en/research/2019/20190423_1.

[1] K. Ohkubo, K. Hirose. Light-driven C-H Oxygenation of Methane into Methanol and Formic Acid by Molecular Oxygen Using Perfluorinated Solvent. Angewandte Chemie International Edition . 2018, 57, 2126-2129. DOI: 10. 1002/anie.201710945
[2] K. Ohkubo, H. Asahara, T. Inoue. Photochemical C–H oxygenation of side-chain methyl groups in polypropylene with chlorine dioxide. Chemical Communications, 2019, 55, 4723-4726. DOI: 10.1039/c9cc01037h
For more information on his work: http://www.irdd.osaka-u.ac.jp/ohkubo/Ohkubo_Lab/Top.html
Text: Saori Obayashi/Edit: Christopher Bubb